Vessel Laminae Segmentation
| Line 50: | Line 50: | ||
==Programs== | ==Programs== | ||
| + | Source code and python script to run the code to be added here. | ||
==References== | ==References== | ||
Arunachalam Narayanaswamy, Saritha Dwarakapuram, Christopher S. Bjornsson, Barbara M. Cutler, William Shain, Badrinath Roysam, Robust Adaptive 3-D Segmentation of Vessel Laminae from Fluorescence Confocal Microscope Images & Parallel GPU Implementation, (accepted, in press), IEEE Transactions on Medical Imaging, March 2009. | Arunachalam Narayanaswamy, Saritha Dwarakapuram, Christopher S. Bjornsson, Barbara M. Cutler, William Shain, Badrinath Roysam, Robust Adaptive 3-D Segmentation of Vessel Laminae from Fluorescence Confocal Microscope Images & Parallel GPU Implementation, (accepted, in press), IEEE Transactions on Medical Imaging, March 2009. | ||
Revision as of 05:02, 27 April 2009
This page describes the vessel surface segmentation algorithm. We provide the background, a brief description of the algorithm and show some sample results. Finally we provide instructions on how to run the program.
Contents |
Background and motivation
Accurate and rapid segmentation of microvasculature from three-dimensional (3-D) images is important for diverse studies in neuroscience, tumor biology, stem-cell niches, cancer stem cell niches, and other areas. It is needed for measuring vascular features such as surface areas, diameters and tortuosities of vessel segments, branching patterns of the vascular tree, and distributions and orientations of cells relative to the vasculature. When time-lapse imaging of living vasculature is performed, segmentation results can be used for analyzing angiogenesis. Finally, quantifying the impact of pharmacological interventions requires vessel segmentation for change analysis.
Vessel segmentation algorithm presents a robust 3-D algorithm to segment vasculature that is image by labeling laminae, rather than lumenal volume. The signal is weak, sparse, noisy, non-uniform, low-contrast, and exhibits gaps and spectral artifacts, so adaptive thresholding & Hessian filtering based methods are not effective.
The Algorithm
The algorithm has four steps
- The first step of our algorithm identifies high-confidence foreground voxels using a robust voxel-based generalized hypothesis test.
- The second step performs an adaptive region growing algorithm to identify additional foreground voxels while rejecting noise. This also yields relative estimates of detection confidence at each voxel.
- The third step uses the marching tetrahedra algorithm to link the detected foreground voxels to produce a triangulated 3-D mesh with watertight isosurfaces. The mesh is smoothed using a volume-preserving algorithm to eliminate jagged facets, and adaptively decimated using an edge-collapsing and volume-preserving algorithm to produce the final mesh. Once this is complete, we estimate the local surface curvature at each triangle.
- Finally, we extract the vessel topology by generating a filled-in vessel that could be traced by tube tracing techniques to produce centerline estimates.
Parameters
For the real data sets used in our experiments, typical values are represented below along with some guidance on how to choose the parameters for a different application.
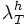 : 8-12
: 8-12
Set this value high enough such that the segmentation detects at least some points in each vessel segment. One way to choose this is to set this equal to 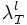 and look at the points detected by the segmentation program. These are the initial points you expect to get detected. Choose this parameter before the lower threshold.
and look at the points detected by the segmentation program. These are the initial points you expect to get detected. Choose this parameter before the lower threshold.
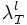 : 3-6
: 3-6
Set this value to a low enough value such that maximum vessel foreground points are detected.
ω : 1.5
This value is half the thickness of the vessels seen in the images(in voxels). Choose this based on the resolution of the imaging involved.
Γ size : 7x7x5
The neighborhood size used for doing hypothesis testing. Higher the neighborhood used, the more robust the test is and the less sensitive it is. Choosing higher neighborhood size also increases the computational time significantly.
Programs
Source code and python script to run the code to be added here.
References
Arunachalam Narayanaswamy, Saritha Dwarakapuram, Christopher S. Bjornsson, Barbara M. Cutler, William Shain, Badrinath Roysam, Robust Adaptive 3-D Segmentation of Vessel Laminae from Fluorescence Confocal Microscope Images & Parallel GPU Implementation, (accepted, in press), IEEE Transactions on Medical Imaging, March 2009.




