The Worm Project
(→Usage of the Code) |
(→Usage of the Code) |
||
| Line 60: | Line 60: | ||
* Background subtraction: For images with cluttered background, it should be set to 1(true). A background image would be computed first; For images with clean background, it can be set to 0(false). Direct threholding and hole filling will be used. | * Background subtraction: For images with cluttered background, it should be set to 1(true). A background image would be computed first; For images with clean background, it can be set to 0(false). Direct threholding and hole filling will be used. | ||
| − | To reach desirable tracking result for some dataset, besides selecting the parameters | + | To reach desirable tracking result for some dataset, besides selecting the parameters the images also could be pre-processed by other methods. For example, when the worms are two small, the image could be resized to make them larger. |
===The Display and Editing Modules=== | ===The Display and Editing Modules=== | ||
Revision as of 18:07, 2 July 2009
The Worm module of FARSIGHT is a toolkit of computational methods designed to segment and track a population of C. elegans worms. The segmentation and tracking data can be used to identify locomotion events (e.g., pirouettes), and to quantify the impact of worm locomotion on external influences (e.g., pheromones). Worms are tracked according to their distance from the pheromone spots and morphological features are computed from the image data for each worm. We present the application of a physically motivated approach to modeling the dynamic movements of the nematode C. elegans exposed to an external stimulus as observed in time-lapse microscopy image sequences. Specifically, we model deformation patterns of the central spinal axis of various phenotypes of these nematodes which have been exposed to pheromone produced by wild type C. elegans Worms are tracked according to their distance from the pheromone spots and intrinsic features are computed from the image data for each worm. The features measured include those proposed by Nicolas Roussel and those provided in the FARSIGHT Toolkit.
A model based algorithm for simultaneously segmenting and tracking of an entire imaging field containing multiple worms previously proposed by Nicolas Roussel has been implemented an applied to model worm behavior. An extension to the work propped by Roussel et. al., is to create a map of the worm’s behavior over time similar to the kymographs used by biologists to improve the accuracy of multiple hypothesis tracking by eliminating hypothesis based on a Euclidean distance metric to the worm kymograph. Interaction between worms is typically modeled as a random walk. We seek to give biologists a new tool to isolate the paths of peristaltic progression of C. elegans leads to unpredictable behaviors that are resolved using a variant of multiple-hypothesis tracking. The net result is an integrated method to understand and quantify worm interactions. Experimental results indicate that the proposed algorithms allow for an integrated high throughput automated analysis of the locomotive behavior of C. elegans during exposure to a given pheromone. The results for this work are generated by a module of the FARSIGHT toolkit for segmentation and tracking of multiple biological systems. The features calculated during this work can be edited and validated using the tracking editor available in the FARSIGHT toolkit. Overall, the method provides the basis for a new range of quantification metrics for nematode social behaviors.
Contents |
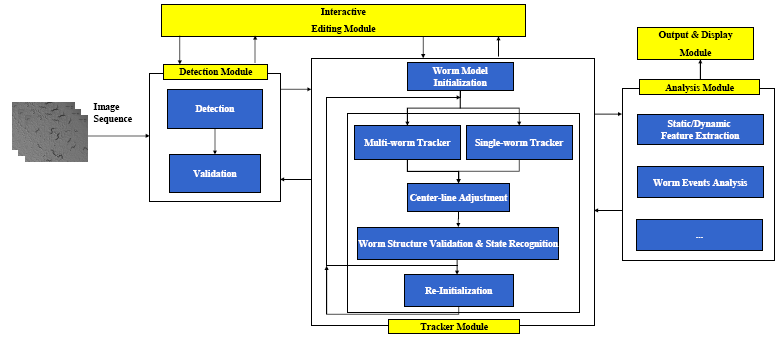
Modules
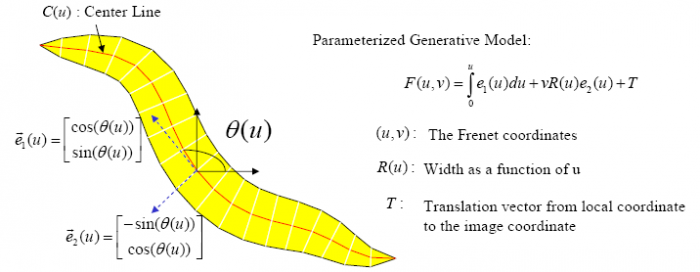
The Worm Model
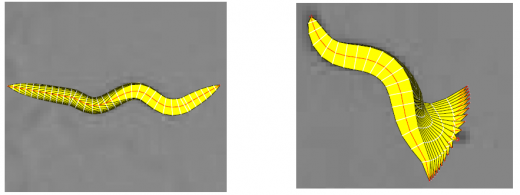
In the tracking system, we combine and modify the two worm models used in [1][2] to make them more suitable for model-based multi-worm tracking, static/dynamic feature extraction and event analysis. This generative model could be deformed to model the two types of worm's crawling movement, as shown in Figure.3.
The Detection Module
The tracker is automatically initialized by the detector. Worm detection generally involves:(1)Binarization and filling small holes (2)worm boundary extraction based on binary image (3)Head and Tail detection (4)Center line and width computation (5)Instantiation of the worm model. In the detection module, detection is followed by the worm model validation to remove errors caused by spots in the background or overlapping worms. The detection is also used for correcting mis-tracked worms during the tracking process. For more details about the Detection Module, please refer to:
The Tracking Module
The framework for the Multi-Worm Tracker is shown in Figure.4. For more details about the tracker, please refer to:
The Analysis Module
The tracking results can be fed to the Analysis Module for static/dynamic feature extraction, and event analysis.
For a complete list of worm features, please refer to
Usage of the Code
For the console program without GUI, usage is as follows:
MWT.exe FileName Index1 Index2 GrayThreshold AreaThreshold1 AreaThreshold2
BGsub OuputDir ImageFormat
FileName: Directory of the images
Index1: First index of the images
Index2: Last index of the images
GrayThreshold: Threshold used for binarization
AreaThreshold1: Minimum object area size
AreaThreshold2: Maximum object area size
BGsub: 1 = ture (compute background image first for images with cluttered background)
0 = false (directly use thresholding and hole filling filter)
OutputDir: Directory for storing the tracking result (a txt file containing point lists, a labeled image, and a image
showing the tracking result)
ImageFormat: Format of the images
Example: MWT.exe E:/WormDemo/TestImages/LGC35ACR20 1 100 40 400 2000 1 E:/ bmp
MWT.exe E:/WormDemo/TestImages/LGC35ACR20 1 100 85 400 2000 0 E:/ bmp
Selection of the parameters: Users need to choose several parameters ralted to the computation of binary images. The quality of binary image is very important for the tracking system.
- Threshold for binarization: When using direct thresholding and hole filling filter for preprocessing, since the intensity of the image could be modeled as a mixture of N=2 Gaussian Mixtures[1], the threshold could be selected as the value separating the two Gaussian distributions, as shown in Figure.5. Intensities smaller than this threshold will be set to 1, the rest will be set to 0.When using background image for subtraction, Intensities larger than the selected threshold will be set to 1, which is different from using direct thresholding and hole filling. The threshold value also can be chosen based on the histogram.
- Minimum and Maximum object area size: They are used to clean the background objects left after binarization. User should try several vaules until reach the desirable binary images.
- Background subtraction: For images with cluttered background, it should be set to 1(true). A background image would be computed first; For images with clean background, it can be set to 0(false). Direct threholding and hole filling will be used.
To reach desirable tracking result for some dataset, besides selecting the parameters the images also could be pre-processed by other methods. For example, when the worms are two small, the image could be resized to make them larger.
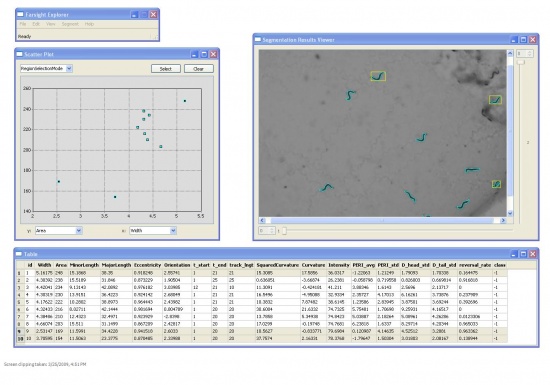
The Display and Editing Modules
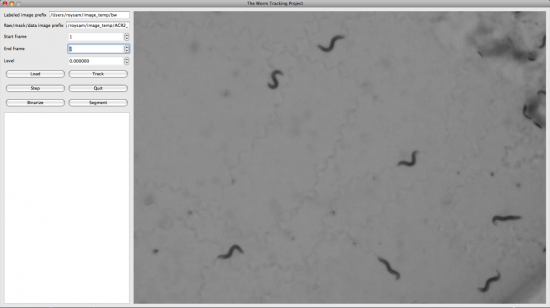
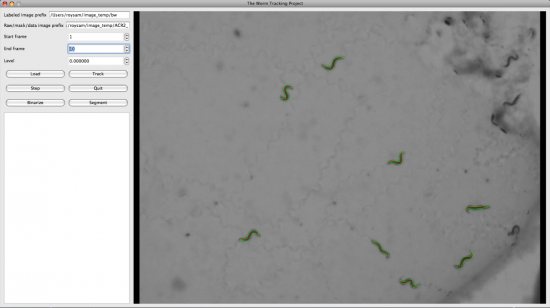
We also developed the Display and Editing modules for the Worm Tracker. The followings are screenshots took from them.
Python Implementation
The worm tracking modules described above have been wrapped in a python script.
In a typical case a user will place images in an image directory
Reference
- [1] Nicolas Roussel, Badrinath Roysam, "A Computational Model for C.elegans Locomotory Behavior: Application to Multiworm tracking", IEEE Trans On Biomedical Engineering. Vol.54, No.10, Oct,2007.
- [2] Fontaine, E., Barr, A., Burdick, J. W., "Model-based tracking of multiple worms and fish", In ICCV Workshop on Dynamical Vision, 2007.
- [3] Fontaine, E., Lentink, D., Kranenbarg, S., Müller, U., van Leeuwen, J., Barr, A.H., Burdick, J. W. "Automated visual tracking for studying the ontogeny of zebrafish swimming", Journal of Experimental Biology, 211, 1305-1316, 2008.
- [4] Nicolas Roussel, "A Computational Model for C.elegans locomotory behavior: Application to Multi-Worm tracking", Phd Thesis, 2007.