Dendritic Spine Segmentation
Contents |
Segmenting Dendritic Spines From 3D Confocal Microscopy Images
Dendritic spines are key to understanding brain cognition and memory as well as drug interaction effects. Their significance has increased in the past decade with advances in 3-D fluorescence microscopy imaging especially for time-lapse analysis (4-D image data) in the context of pre-clinical studies. Segmenting spines is necessary to achieve accurate quantification across several snapshots.
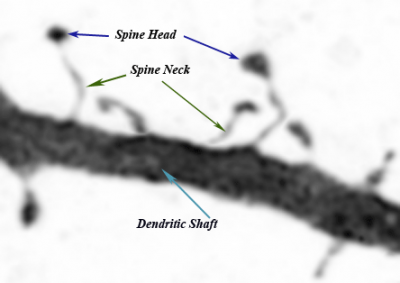
Dendritic Spine Basics
Previously considered insignificant artifacts on the dendritic shaft, the thorn-like protrusions of neuronal membranes first gained attention with Ramon y Cajal’s (1852-1934) novel interpretations of brain tissue images in 1888. Since then, not only the name spines has been retained, but also much of Cajal’s neural spine hypotheses(DeFelipe, J., Ed. (1999) and Garcia-Lopez, P., V. Garcia-Marin, et al. (2006)). Despite the primitiveness of his microscope and techniques, Cajal’s elucidations have laid the foundations of modern neurology (Hamburger, V. (1980)). Spines arise from the neuronal membrane at the soma, dendrites, or the axon hillock in tiny structures and in different directions (Harris, Jensen et al. 1992; Nimchinsky, Sabatini et al. 2002). Their morphologies are quite diverse but are broadly qualified by the head volume and neck diameter. The most commonly used nomenclature categorizes them into(Harris, Jensen et al. 1992; Nimchinsky, Sabatini et al. 2002):
- stubby, closely attached to the dendrite with no obvious neck
- mushroom, has a narrow neck and a large head
- thin, narrow neck and elongated stem with no head
- bifurcated or branched, similar to the mushroom but with a split head.
Fiala et. al.(Fiala and Harris 1999) indicate the dominance of these four types, especially thin-shaped spines, but nevertheless describe a continuum of length and neck deformations. Moreover, specific neurons exhibit additional spine types such as crook thorn and the gemmules.
Significance and 3D Imaging
Topics such as dendritic spine properties (shape, size, distribution, density, …), plasticity, their relationship with the neuronal tree and their role within the synapse are the subject of ongoing research(Yuste and Bonhoeffer 2004; Garcia-Lopez, Garcia-Marin et al. 2006). The computational functionality of the neural dendrite and the role of the spine as the smallest subunit is analyzed in (Sidiropoulou, Pissadaki et al. 2006). Although the complete functions of spines are still not well understood(Yuste and Bonhoeffer 2004), it is widely believed that spines are responsible for information exchange at the synapse. Numerous studies examine dendritic spine correlation with memory(Yuste and Bonhoeffer 2001), cognition and related disorders such as Alzheimer’s Disease(Akram, Christoffel et al. 2007) and fragile X syndrome(Comery, Harris et al. 1997). In recent years, advances in 3D imaging technologies have contributed to increased attention towards investigating spinal structure, development, as well as drug interaction(Harris, Jensen et al. 1992; Nimchinsky, Sabatini et al. 2002; Trachtenberg, Chen et al. 2002; Potter 2005) Age-related spine changes were investigated by (Duan, Wearne et al. 2003) using 3-D confocal images although spine counts were done on 1µm-separated 2-D slices. A recent study on human brain tissue(Akram, Christoffel et al. 2007) found a strong correlation between cognitive decline due to Alzheimer disease and dendritic spine loss. In (Johnson and Ouimet 2004) 3D imaging was used for spine and filopodia length measurements at different time points in order to assess certain drug interactions (protein inhibitors) on spine growth.
Our Methodology
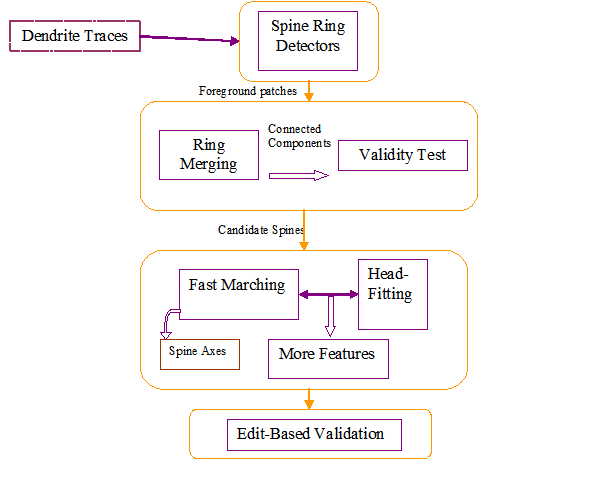
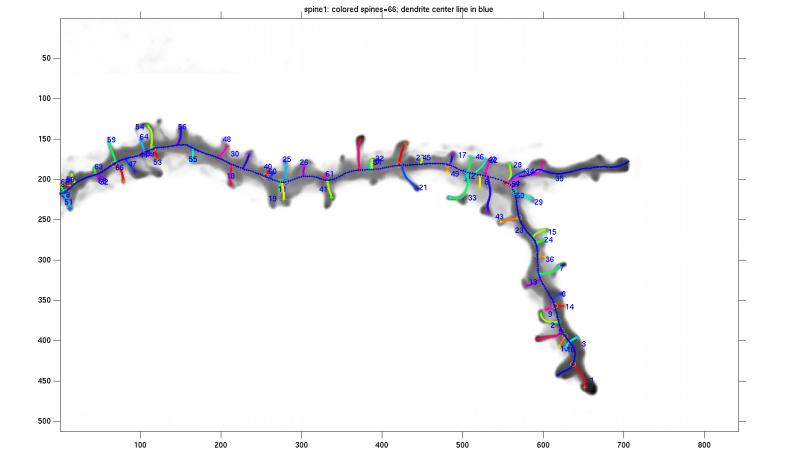
In our algorithm we model the dendritic shaft as a tube-like structure of varying diameter in noise studded with spines, filopodia and/or varicosities. Automated tracing of the dendritic backbone is accomplished by a previously developed algorithm that fits superellipsoids robustly and estimates the local intensity surrounding them. Once this is done, the spines are initially detected by first extracting the surrounding regions that deviate from the tubular geometry. A generalized likelihood ratio test is used to determine whether the initially segmented region represents a spine or just imaging noise. Because spine sizes are close to the achievable image resolution, and fluorescent marker intensity fluctuates within the spine, many spines appear fragmented and/or disconnected from the dendrite and thus require further analysis. Our algorithm sports a novel approach that reconnects apparently detached spines and calculates accurately the spine length using Fast Marching. The spine head, total volume, protrusion from the dendrite surface are features we evaluate for further discriminant analysis.
Spine Ring Detector
The spine ring detector is a 3-dimensional annulus (rectangular toroid) concentric with and orthogonal to the tracing model. The shape of the hollow portion follows the dendrite tracing model. If the dendrite is modeled as a chain of cylinders, the hollow portion is a disc. If the dendrite is modeled by a chain of super-ellipsoids, the hollow portion is a matching super-ellipsoid.
Spine Candidates
We merge the detector responses together, perform a primary validity likelihood test and identify the connected components as spine candidates.
Geodesic Tractography
This is done for two reasons:
- To reattach the apparently detached spines back to the dendrite and therefore identify the most likely spine reconstruction.
- To compute the spine length and distance from the dendrite surface.
We set a fast marching scheme to propagate a wavefront from a starting point on the spine to an ending point on the dendrite. The choice of these points is based on the spine candidate intensity and nearest dendrite center point in the geodesic sense.
Feature Extraction
The spine tip identified in the above step becomes the seed point for fitting a super-ellipsoid to segment the spine head.
Edit-Based Validation Using Farsight
See FARSIGHT_Toolkit and the EVS
How To Run
The steps needed are:
- (Optional) Preprocessing: if the image is too blurry, a deblur filter might be needed. Also too much "salt and pepper" noise might affect the tracing and ring detector output. A median filter might then be needed.
- Dendrite Tracing: Currently our tracing algorithm ALEX TRACING needs to be used. The output feeds into the next step.
- Ring Detection and Initial Candidates module
- Fast Marching Module
- Head Fitting
- Feature Extraction
- Edit-Based Validation
References
- DeFelipe, J., Ed. (1999). Cajal. The MIT encyclopedia of the cognitive sciences. Cambridge, Mass., MIT Press
- Garcia-Lopez, P., V. Garcia-Marin, et al. (2006). "Three-dimensional reconstruction and quantitative study of a pyramidal cell of a Cajal histological preparation." J Neuroscience 26(44): 11249-52
- Hamburger, V. (1980). "S. Ramon y Cajal, R. G. Harrison, and the beginnings of neuroembryology." Perspect Biol Med 23(4): 600-16.
- Harris, K. M., F. E. Jensen, et al. (1992). "Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation." J Neurosci 12(7): 2685-705.
- Nimchinsky, E. A., B. L. Sabatini, et al. (2002). "Structure and function of dendritic spines." Annu Rev Physiol 64: 313-53.
- Fiala, J. C. and K. M. Harris (1999). Dendrite Structure. Dendrites. G. Stuart, N. Spruston and M. Häusser. Oxford, UK, Oxford University Press.
- Yuste, R. and T. Bonhoeffer (2001). "Morphological changes in dendritic spines associated with long-term synaptic plasticity." Annu Rev Neurosci 24: 1071-89.
- Yuste, R. and T. Bonhoeffer (2004). "Genesis of dendritic spines: insights from ultrastructural and imaging studies." Nat Rev Neurosci 5(1): 24-34.
- Sidiropoulou, K., E. K. Pissadaki, et al. (2006). "Inside the brain of a neuron." EMBO Rep 7(9): 886-92.
- Akram, A., D. Christoffel, et al. (2007). "Stereologic estimates of total spinophilin-immunoreactive spine number in area 9 and the CA1 field: Relationship with the progression of Alzheimer's disease." Neurobiol Aging
- Comery, T. A., J. B. Harris, et al. (1997). "Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits." Proc Natl Acad Sci U S A 94(10): 5401-4
- Trachtenberg, J. T., B. E. Chen, et al. (2002). "Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex." Nature 420(6917): 788-94.
- Potter, S. M. (2005). Two-photon microscopy for 4D imaging of living neurons. Imaging in Neuroscience and Development: A Laboratory Manual. K. A. Yuste R, Cold Spring Harbor Laboratory Press: 59-70
- Duan, H., S. L. Wearne, et al. (2003). "Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys." Cereb Cortex 13(9): 950-61.
- Johnson, O. L. and C. C. Ouimet (2004). "Protein synthesis is necessary for dendritic spine proliferation in adult brain slices." Brain Res 996(1): 89-96.
- Tyrrell, J. A., E. di Tomaso, et al. (2007). "Robust 3-D modeling of vasculature imagery using superellipsoids." IEEE Trans Med Imaging 26(2): 223-37.